Hi All
Interesting report, here is what they have to say on reagent production:
Test execution
Two main challenges have led to a limited capacity to execute tests: a shortage of the laboratory equipment and trained personnel needed to run tests and a shortage of the necessary reagents, which are often packaged as kits (testing kits and RNA-extraction kits, for example).
Building and installing new equipment takes time—between 20 and 30 days for an order of high-throughput equipment to be delivered, for instance, and at least three to five days for it to be installed, calibrated, and validated for diagnostic testing.6 Newly installed equipment also requires more trained personnel to operate it. Moreover, financial constraints in many countries—government funding for public health laboratories, for example—can make it hard to build additional capacity.
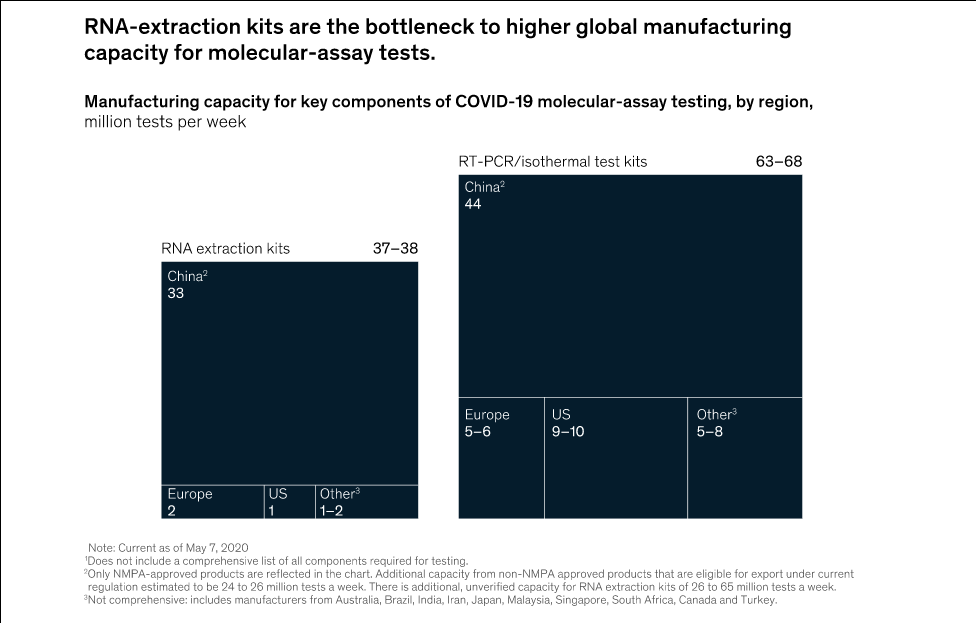
Executing a test requires some 20 different reagents, consumables, and other pieces of equipment. Of those materials, major shortages have been reported in RNA-extraction kits and certain reagents, including enzymes and primers.7 The global manufacturing capacity for molecular-assay tests is estimated to be between 37 million and 38 million tests a week, given current availability of the various test components, with RNA-extraction kits being the bottleneck to higher capacity (Exhibit 3).8 That compares with fewer than 10 million tests a week being conducted around the world, according to our research.
There are two potential explanations for the gap. First, a significant quantity of the reagents being manufactured are those that run on open systems—that is, less integrated systems that can run a wider range of test methods. Such reagents cannot be used with the high-throughput machines that tend to be used in developed countries and are closed systems requiring cartridges loaded with proprietary reagents manufactured by the OEM. Second, as Exhibit 3 shows, most of the available manufacturing capacity is based in China, potentially making access to it more difficult, given validation and export considerations.